 |

|
 |

|
 Back to 2001 3rd Quarter Table of Contents
Back to 2001 3rd Quarter Table of Contents
Introduction Hepatitis C is a major Public Health threat and of major concern to all Health Care Practitioners because of the prevalence and long term consequences of the disease. It is the most common U.S. blood borne infection, and affects close to four million people. This is approximately four times the number of HIV infected individuals.1 From a Chiropractic and Holistic Practitioner’s position, Hepatitis C infected individuals are very likely to exist in their patient populations. The Hepatitis C patient typically will present with a variety of functional but not necessarily overt symptoms that span multiple organ systems. The hepatic damage is due to both the cytopathic effect of the virus and the inflammatory changes secondary to immune activation.2 Accurately recognizing the source of these problems allows referral of the patient for appropriate diagnosis. Informing a positively identified Hepatitis C patient of their options is an important aspect of improving quality of life and increasing clinical success. Hepatitis C is the most common cause of chronic liver disease, cirrhosis and hepatocellular carcinoma in the Western World. Chronic Hepatitis C is asymptomatic 95% of the time. It is estimated that more than 30% of patients with chronic Hepatitis C develop cirrhosis. Thus, this liver disease can lead to end-stage liver disease despite the presence of few symptoms and signs of illness. Functional Assessment of Patients Because of its chronic, sub-clinical character, Hepatitis C often goes undiagnosed. In my clinical opinion, it is more effective to identify subjective symptoms and risk factors (see Tables 1,2, below) in determining the possibility of Hepatitis C infection.3 Any patient or client with these risk factors, and especially if they have functional and viscero-somatic signs and symptoms of liver involvement, is a good candidate for Hepatitis C screening since 70% of chronic carriers are asymptomatic. When symptoms are present, the most common are fatigue (70%), abdominal pain/discomfort (20%), anorexia (15%) and weight loss(5%). The majority of chronic HCV carriers have hepatomegaly (70%) while some have an enlarged, palpable spleen (20%). Only 25% of acute HCV cases Table 1: Routes of transmission IV-Drug User .................................... 45%Sex/Household ................................ 15%Unknown .......................................... 40% Table 2: Risk factors Blood Transfusions Hemodialysis Treatment IV-Drug User Tattoo Needle-stick and sharps injuries Sexual contact where partner has severe hepatitis C (only 5% of cases) Saliva (speculative) contamination from infected person Anyone exposed to blood products develop jaundice.4 From a functional analysis, because Hepatitis C attacks the liver, there are sub-clinical systemic multi-system complications. For example, the conversion of B-vitamins occurs in the liver and secondary impairment can lead to changes in circulating hormone levels, decreased ability to detoxify and neurological manifestations. Non-specific signs and symptoms may include pain over the rib cage on the right side, back and right shoulder pain, itchy skin, alternating stool color, fluid retention, foul body odor, sclera of eyes appear yellow, easy bruising, halitosis, migraine (traditionally seen in botanical medicine as a liver issue) aching muscles not due to exertion. Often the patient with a poorly functioning liver will have symptoms of sluggish digestion, fat intolerance, flatulence, bloating, nausea, chronic constipation and chemical, food or drug intolerances.5 Obviously, secondary to somato-visceral and visceral-somatic reflexes, many of these patients will seek Chiropractic care as they often complain of sub-clinical neurological and diffuse musculoskeletal symptoms. Therefore, it is imperative to use a general holistic health screening questionnaire in the office. If you want to see the whole patient, you must assess the whole patient. Many nutritional companies provide general screening questionnaires that provide a subjective analysis of all major organ systems. Conventional and Alternative Options Despite progress in detecting and treating Hepatitis C at present, there are no specific non-toxic and effective proven treatments for Hepatitis C. Alpha interferon is the only FDA-approved therapy for chronic Hepatitis C. Interferon treatment, however, is expensive and often poorly tolerated due to its numerous side effects and results in beneficial long-term responses in only a minority of patients.6 Failure to respond to Alpha Interferon occurs in 40-60% of patients, 15-45% relapse and only 15-25% show a positive response. The side-effect profile of interferon Alpha Interferon (alfa2b) is high and includes nausea, headache, fever, myalgias, fatigue, leukopenia, thrombocytopenia, alopecia, irritability, depression, thyroid abnormalities, pulmonary complications, and retinal damage.7 Patients not responding to treatment with interferon after three months, or who experience serious side effects, are not good candidates for continued interferon therapy. Unfortunately, there have been very few alternatives which could provide objective improvement in clinical indicators and symptoms and that were non-toxic. Recently, a proprietary blend of three Korean and Chinese botanicals have been introduced into the U.S. Market8 that has shown in pilot clinical trials to be non-toxic and provide substantial clinical and symptomatic resolution of Hepatitis C9 (Table 3, p. 175). In this study, 6 males and 4 females between the ages of 41 and 68 (avg. 54.4 yoa), all diagnosed as positive for Hepatitis C antibodies with elevated liver enzymes (ALT/GPT) were given a proprietary blend of herbs. The preparation consisted of two 500 mg capsules of 3 herbs given twice daily for 24 months. All patients showed a good response and their viral load (HCV-RNA) levels gradually lowered from one-billion or one-hundred million to one thousand copies/ml serum during the study. In other words, viral loads decreased 1,000,000 to 100,000 times. Patients in the study also reported improved quality of sleep, increased physical energy, improved countenance, increased body weight and smoother and softer skin. Non-toxicity was confirmed by a separate analysis by the Institute of Life Sciences and Chungbuk National University in Korea. Their results concluded that “. . oral administration [of a proprietary herbal formula] for 28 days, even at 20 times higher human therapeutic doses, does not cause any adverse effect to the body.” This blend of three traditional botanicals contains Patrinia Villosa, Artemesia Capillaris, and Schizandra fructus. The three herbs in this formula have been shown to be hepatoprotective, support the regeneration of liver cells, increase the flow of bile, lower bilirubin and cholesterol levels and to be anti-viral.10-13 Quality and Safety of Botanical Products If your patient decides to utilize complementary treatment or experiment with herbal support for Hepatitis, or any other condition, it is essential to counsel them on the differences in quality between herbal products. Many herbal products are contaminated with heavy metals, pesticides, bacterial or mold/fungus, therefore it is important to establish a relationship with quality herbal manufacturers as these contaminants can exacerbate hepatic damage by further stressing an already under-functioning liver.14-16 Summary Hepatitis C is a serious and common problem that practitioners are likely to encounter. The disease often lacks overt signs and symptoms and, therefore, often evades diagnosis. It is imperative to “dig deeper” when evaluating a patient coming in with functional disturbances that could possibly be linked to the liver, especially when those disturbances are accompanied by one or more exposure risk factors. Until now, if a diagnosis of Hepatitis C is made, there have been no effective remedies that were safe, well-tolerated and effective. Preliminary evidence on a proprietary blend of Korean and Chinese Herbs now available in the U.S. for remediation of Hepatitis C is very promising and appears to be a worthy of further investigation. References Table 3. Changes in HVC RNA during the botanical therapy. HVC RNA levels gradually reduced from 1X108 or 1X109 to 1X103 with oral administration of herbal formulation. 109 108 107 106 105 104 103 102  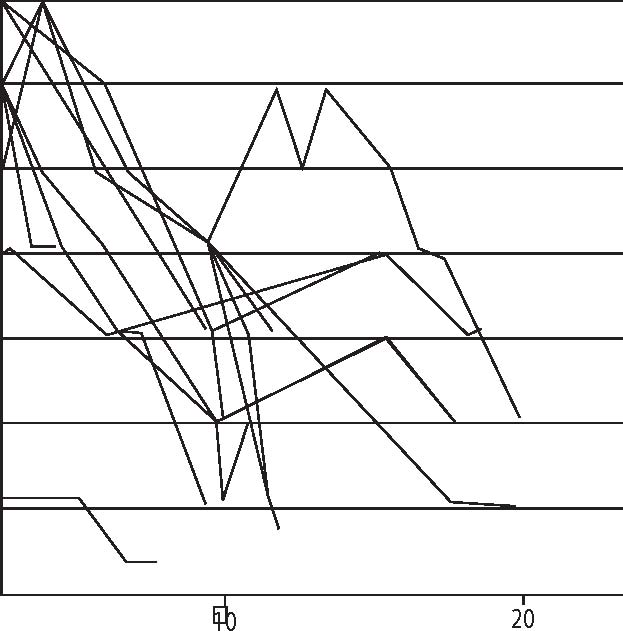 Treatment period in months
Winter, 2000: www.nutricology.com/Newsletter/eurocel.htm 10.The Review of Natural Products by Facts and Comparisons. St. Louis, MO: Wolters Kluwer Co., 1999. 11. Liu GT: Pharmacological actions and clinical use of fructus Schizandrae. Chin Med J, 1989; 102: 740-749. 12. Li XY: Bioactivity of neolignans from fructus Schizandrae. Mem Inst Oswaldo Cruz 1991; 86: 31-37. 13.Stern E: Two Cases of Hepatitis C Treated with Herbs and Supplements. J Altern Compl Med, Vol. 3 (1), 1997, 77-82. 14.Moore C, Adler R: Herbal vitamins: lead toxicity and developmental delay. Pediatrics, 2000 Sep; 106(3): 600-2
|

This website is managed by Riordan Clinic
A Non-profit 501(c)(3) Medical, Research and Educational Organization
3100 North Hillside Avenue, Wichita, KS 67219 USA
Phone: 316-682-3100; Fax: 316-682-5054
© (Riordan Clinic) 2004 - 2024c
Information on Orthomolecular.org is provided for educational purposes only. It is not intended as medical advice.
Consult your orthomolecular health care professional for individual guidance on specific health problems.