Abstract The Hardin Jones-Pauling biostatistical theory of survival analysis for cancer patients leads naturally to three criteria for the validity of clinical trials of treatments for cohorts of cancer patients. These three criteria are presented. The well-publicized Mayo Clinic study claiming the lack of efficacy of vitamin C in prolonging the lives of patients with advanced colorectal cancer is shown to violate each of the three criteria. A detailed account is given of the reprehensible treatment received by Linus Pauling and the author from the New England Journal of Medicine when they attempted to publish their analysis of the Mayo Clinic study in the New England Journal of Medicine. 1. Introduction In a paper recently published in this journal,1 the salient details of the Hardin Jones-Pauling biostatistical theory of survival analysis for cancer patients were presented. The advantages of this theory over the conventional analysis were illustrated by means of an example of a clinical trial taken from the literature. The basic idea of the Hardin Jones-Pauling theory of survival analysis for cancer patients is that, for a given type of cancer and particular therapy (or no therapy), survival as a function of time for a homogeneous cohort of patients 1. 521 Del Medio Avenue, #107 Mountain View, CA 94040 USA E-mail: zelek.herman@forsythe.stanford.edu is well approximated as a first-order reaction, so that the rate of change of the survival function Sˆ(t) has the characteristic behavior dˆS(t) =kt , (1) dt where k is the rate-of-death constant. This concept has been given the appellation the Hardin Jones Principle. In this equation, S ˆ(t) may be computed by employing the conventional Kaplan-Meier normalization procedure.2,3 Integration of equation (1) leads to ˆ(t)S Sˆ(0) = exp(-kt) , (2) where Sˆ(0), (2) is the value of the Kaplan-Meier Product-Limit survival function at time zero. Then, taking the natural logarithm of both sides of equation (2) results in ln[Sˆ(t)/Sˆ(0)] =-kt (3) or lnSˆ(t) = lnSˆ(0) – kt , (4) which is the equation of a straight line with slope -k and ordinate intercept Sˆ(0). Thus, a graph of the natural logarithm of the Kaplan-Meier Product-Limit survival function Sˆ(t) as a function of time will be a straight line with slope equal to -k and ordinate intercept Sˆ(0) at t = 0. Moreover, half of the patients will be alive (i.e., Sˆ(tH) = 0.5 Sˆ(0)) at time equal to tH (the half-life), or t1  = ln(0.5) ≈ 0.693147 (5) = ln(0.5) ≈ 0.693147 (5) 2kk Alternatively, one may use the relationship of the natural logarithm to the common logarithm (ln(x) = ln(10)•log(x) — 2.30259 • log(x)) to plot the common logarithm of the survival function of time. In this case, the ordinate intercept will occur at the value 2, which is the common logarithm of 100%. Again, this plot is a straight line but with slope now given by -k/2.30259. For the case of two homogeneous cohorts, then, the survival function is well approximated by the sum of two first-or-der reaction curves, with independent rate constants k1 and k2. For details, the reader is referred to [1]. A more sophisticated analysis1,4 using the Hardin Jones-Principle enables one to calculate the mean survival time 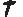 according to the equation according to the equation 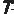 = =
 1.7810724 exp <lnt > + 1.7810724 exp <lnt > +
 (6) (6)
< t2 > + 4[< t1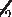 2 >]2+t1 2 >]2+t1
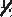 2 π 2 2 π 2
For N0 members in a cohort at the beginning of the trial, the mean deviation from  is somewhat less than 1/N0. An example of the application of the Hardin Jones-Pauling biostatistical theory of survival analysis to a published clinical trial of patients with acute myelogenous leukemia is presented in [1]. is somewhat less than 1/N0. An example of the application of the Hardin Jones-Pauling biostatistical theory of survival analysis to a published clinical trial of patients with acute myelogenous leukemia is presented in [1]. Using as a starting point the Hardin Jones Principle that the death rate of members of a homogeneous cohort of cancer patients is constant, it is possible1,5 to formulate three criteria for the validity of clinical trials of cancer patients: I. The treatment of all of the members of the cohort should be the same, and it should be continuous and unchanged from the time t = 0 when the patient enters the trial until the time t when the patient dies or t+ when, without dying from the malignancy, the patient is withdrawn from the set of survivors at risk. Any patient who stops the treatment, changes the treatment, or dies from a cause unrelated to the malignancy at any time t+ should be removed from the study and included in the analysis by means of the Kaplan-Meier renormalization procedure2 or the alternative procedure provided by the Hardin Jones-Pauling biostatistical theory of survival analysis.1 If the trial is to test the later response of patients to a short-term course of treatment (with or without following a continuous course of treatment), the time t = 0 is to be taken as the time at which the short-term course was completed, with only those patients who survived the short-term course included in the analysis. II. A meaningful clinical trial of the survival of cancer patients must be characterized by having the semilogarithmic plot of survival versus time have a line that passes through Sˆ= 100% at t = 0. No statistically significant lag period in which no deaths happen can occur. A clinical trial producing a set of survival times with a significant lag period from t=0, during which period the value of the death constant k would lead to the expectation that several deaths would occur, can be considered to be faulty. III. If the cohort is heterogeneous and the investigation properly carried out, with conditions constant during the period of the trial (the first criterion), then the semilogarithmic survival curve must bend away from the Hardin Jones initial straight line only in the direction of increased survival times for the longer-term survivors. Of course, this is to be expected since the subcohort of patients with shorter life expectancy is depleted, leaving the subcohort with longer life expectancy. The observation that the semilogarithmic plot of survival versus time bends downward as time increases indicates faulty design or execution of the clinical trial. This situation can occur if, after the death of many or most of the patients in the subcohort with high death rate, some of the survivors might have had their treatment changed in such a way as to increase the death rate. According to the first criterion, these patients should at the time of change of treatment been removed from the study. Linus Pauling and the author examined more than 200 studies of survival of cohorts of cancer patients and found that these three criteria are satisfied. However, we did find a single reported clinical trial that fails on each of these criteria for validity. This study is the subject of this paper. 2. The Mayo Clinic Study of the Efficacy of Vitamin C in Prolonging the Lives of Patients with Advanced Colorectal Cancer -An Example of a Clinical Trial that Fails to Meet All Three Criteria for the Validity of Clinical Trials In 1985, Charles Moertel and his associates at the Mayo Clinic in Rochester, Minnesota, published a paper that describes a clinical trial purporting to investigate the possible efficacy of vitamin C in extending the lifetimes of patients with advanced adenomatous colorectal cancer.6 This investigation was carried out to check the claims of Pauling and Cameron that high-dose vitamin C might be of value in the treatment of advanced cancer.7 Even though the researchers at the Mayo Clinic investigated only patients with advanced adenomatous colorectal cancer, they concluded as a result of their study that “high-dose vitamin C therapy is not effective against advanced malignant disease regardless of whether the patient has had any prior chemotherapy.” (Cameron and Pauling had already criticized as faulty an earlier study by Moertel and his coworkers of vitamin C and cancer on patients who had received prior chemotherapy on the grounds that chemotherapy severely weakens the immune system.) The results of the trial received a great amount of publicity in the popular press, with headlines appearing that stated such things as, “Vitamin C of No Value in Treating Cancer.” Pauling and I analyzed this study and found that it fails on each of the three criteria for the validity of clinical trials of cohorts of cancer patients discussed in the preceding section. This study by Moertel et al., was described in their paper as a randomized double-blind* comparison of the administration of either vitamin C in the amount of 10 grams per day (the test group) or a lactose placebo (the control group) to 100 patients with advanced adenomatous colorectal cancer who had not received any prior chemotherapy. In their paper, Moertel et al. gave survival times only in a Kaplan-Meier survival figure. When Pauling and I requested the data from each of the investigators, they, in very unscientific responses, refused to provide us with any information beyond that published in their paper. Fortunately, by carefully measuring the vertical changes in the Kaplan-Meier graph and comparing these values with the number of patients at risk (realizing that each value must correspond to an integral number of patients), we were able to extract the survival times and employ them to plot the Hardin Jones semilogarithmic graph shown in Figure 1. (p.228) It should be pointed out that it is, in general, not possible to uniquely extract such data from a Kaplan-Meier graph. *A double-blind study is one in which neither the investigators nor the patients know the treatment being administered. “Randomized” means that patients in the treatment and control groups have been matched as closely as possible with regard to age, gender, condition, etc. and then randomly assigned to one of the two groups. In this study conducted by Moertel and his associates, each of the 51 vitamin C patients received vitamin C for some time (the paper states the median time of administration was 2.5 months). The vitamin C was stopped for 19 patients with clearly measurable areas of malignant disease when there was a 50% increase in the product of the perpendicular diameters of any of these areas and, for the other 32 patients, when there was some other evidence of progression of the disease. Each of these 51 patients then entered a period of no treatment, during which some of them died. Then 30 of the vitamin-C patients entered a third period after they “discontinued participation in the study,” according to the authors. During this period, they received chemotherapy, mostly with 5-fluorouracil (a debilitating cytotoxic drug previously shown by Moertel and his colleagues to be of little value in treating patients with advanced colorectal cancer) either alone or in combination with other chemotherapeutic agents. Thus, there were four periods for the vitamin-C patients: the period of regular intake of vitamin C, the following period of no treatment, the period of chemotherapy, and the final period after the cessation of chemotherapy. Although the authors treat the data as though it were a single clinical trial, it was, in effect, four trials, and it clearly violates the first criterion for the validity of clinical trials of treatments of cohorts of cancer patients, which requires that the treatment remain the same throughout the course of the trial. As can be seen in Figure 1, the second criterion of validity is also violated in that the Hardin Jones initial straight line does not intercept the ordinate at a value near 2 (corresponding to 100%). Pauling and I were not able to explain the lag period of about 70 days, during which only one patient died, rather than about 25, as expected from the slope of the Hardin Jones line. We were also not able to make a good biostatistical analysis of the death rates under the four separate conditions (vitamin Figure 1. Logarithm of the Kaplan-Meier Product-Limit survival percentage Sˆ for advanced colorectal cancer patients for two cohorts (vitamin C and placebo) as a function of the time t from the onset of treatment (data from [6]). 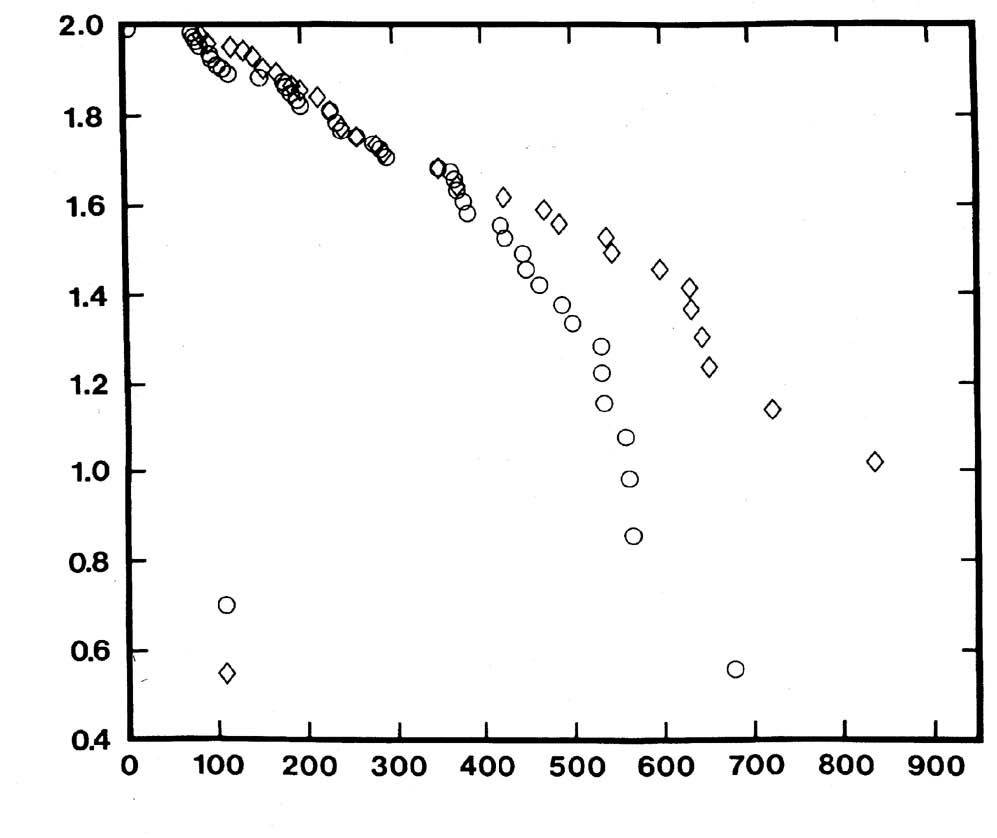 Time (days) The Application of the Hardin Jones-Pauling Biostatistical Theory of Survival Analysis
C or placebo being taken, no treatment, chemotherapy administered, and then no treatment) because the authors refused to provide us with information concerning the time periods during which the individual patients were in each of these four periods. Since none of the vitamin C patients died while taking the vitamin one conclusion is certain, namely, that this study provides no information about the value of a continued intake of 10 grams per day of vitamin C in extending survival time for patients with advanced adenomatous colorectal cancer. This study by Moertel and his associates also clearly violates the third criterion for the validity of clinical trials of treatments of cohorts of cancer patients. It is immediately obvious from Figure 1 that the Hardin Jones plots curve downwards for the vitamin C group at times greater than about 400 days and for the control group at times greater than about 600 days. The reason for this may be that the life expectancy of the patients alive at these times was decreased somewhat by chemotherapy. Moreover, even though this study claims to test the earlier work by Cameron and Pauling,7 Moertel and his coauthors apparently did not bother reading Cameron and Pauling’s book, where on p. 117, it is pointed out that the sudden cessation of intake of high doses of vitamin C leads to a rebound effect that could be dangerous for cancer patients. (The rebound effect is often manifested in healthy people taking large doses of vitamin C by the occurrence of a cold when they suddenly stop the vitamin C, and at the same time, are subject to stress or travel in an airplane.) In the Moertel study, during the period from 70 to 120 days, ten vitamin C patients died, whereas only four placebo patients died during this period. Also, the reported death rate for the vitamin C patients was significantly greater during the following 300 days. This increased death rate occurred immediately after the median-reported cessation of vitamin C intake, 75 days. This suggests that the increased death rate for the so-called vitamin C patients during this period may, in fact, have been caused by the rebound effect. The administration of chemotherapy to 58 patients who had withdrawn from this study had begun by 250 days, when 58 patients were still alive. However, the death rate increases at about 400 days for the “vitamin C patients” and at about 600 days for the lactose patients. According to the Hardin Jones Principle, this means that something had changed in the treatment or environment of the patients, causing them to die at an increased rate, and the administration of chemotherapy seems to be a likely cause. A further, and obvious, criticism of this study is that the choice of placebo, namely lactose, for the control group was a poor choice in a trial that is supposedly double blind. Indeed, lactose is a sugar while vitamin C (L-ascorbic acid) is a weak acid. A better choice of placebo would have been a mixture of lactose and citric acid that mimics the taste of vitamin C. Insofar as the Mayo Clinic is concerned, it is interesting to mention that, in 1989, the Biochemistry and Molecular Biology Graduate Students Association of the Mayo Medical School invited Professor Pauling to travel to Rochester, Minnesota to speak to them about the Moertel paper. It speaks well for the administrators of the Mayo Medical School that they did not overrule this invitation. Over Labor Day weekend of that year, Linus Pauling and I flew to Rochester. On 5 September, Professor Pauling presented two lectures at the Mayo Clinic to overflow audiences in a large lecture hall. The first lecture was held in the morning, when Pauling gave a talk on “The History of the Determination of Protein Structures.” The second lecture was held in the afternoon, and it dealt with the Hardin Jones-Pauling biostatistical theory. Both lectures were videotaped by the audiovisual staff at the Mayo Clinic, and they promised to send me copies of the videotapes. After a period of some months, I had still not received these videotapes, and I called to request them. The Mayo Clinic then kindly sent me a videotape of the morning lecture, but they also informed me that all copies of the afternoon lecture had been erased and used for other purposes. Fortunately, I had been allowed to make my own audiotapes of both lectures using crude equipment, and I still possess these tapes. In addition, when our hosts at the Mayo Clinic, Charles Crutchfield and Robert Horton, accompanied Pauling and me to the Rochester airport for our return trip to California on 6 September 1989, they informed me that Dr. Moertel was in the waiting area for the first leg of our trip, from Rochester to Minneapolis-St. Paul, and that he was traveling on the same flight. After we boarded, I asked a flight attendant to deliver to Dr. Moertel a note stating that Professor Pauling would be interesting in speaking with him. The flight attendant returned shortly afterward, and she informed Pauling and me that Dr. Moertel did not wish to speak to Professor Pauling. Some years later while at a conference, I met a physician colleague of Dr. Moertel’s from the Mayo Clinic, and he told me that Dr. Moertel (who was a heavy smoker, as was Dr. Cameron, even though he was an oncologist) had recently died from leukemia while still in his 60s. 3. A Sordid Experience with the New England Journal of Medicine The New England Journal of Medicine has a reputation for being one of the premier medical journals in the world. The experience Linus Pauling and I had with this journal in attempting to publish a manuscript dealing with the topic discussed in the preceding section indicates that this reputation was not deserved under aegis of its previous editor, Dr. Arnold S. Relman. In the early part of 1985, Linus Pauling and I prepared a manuscript entitled “An Analysis of a Randomized Double-Blind Study of the Effects of Giving Vitamin C to Patients with Advanced Colorectal Cancer and then Stopping the Vitamin C and Administering Chemotherapy,” and we submitted it to be considered for publication in the New England Journal of Medicine. The paper was written in response to the much-publicized paper by Charles Moertel and his associates6 that appeared in the 17 January 1985 issue of the New England Journal of Medicine. As mentioned previously, these authors claimed, on the basis of the clinical trial summarized in this paper, that vitamin C is of no value in extending the lifetimes of patients with advanced cancer. Following the submission of our manuscript to the New England Journal of Medicine, Pauling and I received from this journal the customary response, dated 26 April 1985, and signed by the editor, Dr. Arnold S. Relman, informing us that our manuscript had been received at the journal office and that we would be notified “of the Journal decision as soon as possible.” Moreover, Dr. Relman’s letter stated that the “Journal undertakes review with the understanding that neither the substance of the article nor any of the figures or tables have been published or will be submitted for publication elsewhere during the period of review.” Pauling and I waited patiently for a “soon as possible” response telling us whether or not our manuscript would be accepted for publication, but after 15 months of waiting for a decision, our patience was exhausted. Consequently, on 29 July 1986, we wrote to Dr. Relman, reminding him that, “During the time since we submitted our manuscript we have adhered to the policy that you advocate, in that we have not submitted the manuscript or any part thereof to another journal.” Furthermore, we asked Dr. Relman to now “make a decision about our manuscript, without further delay, either accepting it or rejecting it. We hope that you will decide to accept it for publication in the New England Journal of Medicine.” No response was forthcoming from Dr. Relman. On 17 November 1986, Pauling and I wrote to J. F. McDonaugh, Chairman, Committee on Publications, the Massachusetts Medical Society, publishers of the New England Journal of Medicine. In our letter to Dr. McDonaugh, we informed him of the situation and asked him to facilitate a decision. Again, no reply. On 7 February 1987, Pauling wrote again to Relman, repeating our request for a decision regarding the publication of our manuscript. Again, no response. On 25 February 1987, Pauling wrote another letter to Relman, stating in part: I think that you should make a decision about publication or rejection of the paper that Dr. Zelek S. Herman and I submitted to you, as editor of the New England Journal of Medicine, nearly two years ago. I must say that I consider it ungentlemanly for you not to answer my letters; but I was brought up in Oregon, not in New England, where the code of gentlemanly behavior may be different. As usual, there was no response. Frustrated by our attempts to get a decision from Dr. Relman, Professor Pauling asked the lawyer of the Linus Pauling Institute to write to Dr. Relman. Very soon after this letter was mailed, a response was finally received on September 4, 1987: Dear Dr. Pauling: Your lawyer’s letter served to remind me that although we had made the decision a long time ago not to publish your manuscript, “An Analysis of a Randomized Double-Blind Study of the Effects of Giving Vitamin C to Patients with Advanced Colorectal Cancer and then Stopping the Vitamin C and and [sic] Administering Chemotherapy” we had never written to explain the reasons why–or even to give you formal notice of our decision. For that lapse I apologize. However in my defense I must point out that I had long ago made it perfectly clear to Dr. Cameron, your collaborator, and I think to you, that we did not feel we could consider any further articles on Vitamin C therapy for cancer unless they were supported by new and convincing data obtained from controlled clinical trials. Relman’s letter concluded with a list of reasons for deciding not to publish the paper. The reader should be cognizant of the fact that it is within the perogative of a journal to accept or reject a manuscript submitted for publication. However, it is perfectly clear that Dr. Relman wanted us to withdraw our request for publication. By his actions, he, in effect, prohibited the timely analysis of what we considered to be a fraudulent paper. After all this time had passed since the submission of manuscript, we decided to write a substantially revised one and published it in 1989.5 This was not an isolated case of suppressing the truth by the New England Journal of Medicine. Indeed, Robert Horton, one of our hosts at the Mayo Clinic, informed us that he had submitted a letter to the editor of the New England Journal of Medicine criticizing the Moertel paper for not considering the rebound effect. According to him, Horton’s letter was rejected for publication in the New England Journal of Medicine because his point of view was “adequately represented” among the other letters received on this subject and “already accepted for publication.” None was ever published in the New England Journal of Medicine. In addition, Dr. Ewan Cameron had submitted to the New England Journal of Medicine a manuscript containing an analysis of his clinical trials on the effectiveness of vitamin C in prolonging the lifetimes of terminally ill cancer patients. He was told by the New England Journal of Medicine that he needed to revise this manuscript to make it suitable for publication there. After carefully revising his manuscript in accordance with the suggestions of the referees, he received the same response. Finally, he gave up and published a summary of the results of his findings elsewhere.8 This whole episode is nothing short of scandalous. One wonders if Linus Pauling, the only person to have received two unshared Nobel prizes and who made many important medical discoveries (to name but a few: the alpha helix; the clarification of how oxygen bonds to hemoglobin; with his colleague, Dr. Harvey Itano, the cause of sicklecell anemia; the invention of the Pauling oxygen meter, which saved the sight of countless premature infants) could have his work repressed by seemingly reputable authorities, what chance would people of less stature have in combatting the medical establishment? In retrospect, it is ironic that many of Dr. Pauling’s ideas concerning vitamin C now have been quietly accepted by many practicing physicians, who routinely advise patients to take it. An even-handed and accurate analysis of the cancer and vitamin C controversy was published by Evelleen Richards, a Senior Lecturer in the Department of Science and Technology Studies at the University of Wollongong, Australia, as a book bearing the title “Vitamin C and Cancer: Medicine or Politics?”9 This book is highly recommended to readers interested in the sordid history described above. Dr. Richards concludes in her book that, “The vitamin C and cancer controversy is clearly not over. It continues with its familiar mix of medicine and politics.” 4. Conclusions Three criteria for the validity of clinical trials of cohorts of cancer patients result from the Hardin Jones-Pauling biostatistical theory of survival analysis for cancer patients. It has been demonstrated that the Mayo Clinic trial purporting to test the efficacy of vitamin C in extending the lifetimes of patients with advanced colorectal cancer fails to meet each of the three criteria. Finally, the sordid treatment received by Linus Pauling and the author by the New England Journal of Medicine, when they attempted to publish their analysis of this faulty trial in the New England Journal of Medicine, which published the original study, indicates that medical research is not always the noble enterprise it is perceived to be. It is hoped that this presentation will help lead in some small way to the rational judgment of medical claims. Acknowledgments The author thanks Dorothy Bruce Munro and Jane Priddy Buechel for proof-reading the manuscript. References - Herman ZS: On understanding the Hardin Jones-Pauling biostatistical theory of survival analysis for cancer patients. J Orthomol Med, 1998; 13: 141-160.
- Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc, 1958; 53: 457-481.
- Miller, Jr., RG: Survival Analysis. New York. John Wiley & Sons. 1981; 238 pages.
- Pauling L: Biostatistical analysis of mortality data for cohorts of cancer patients. Proc Natl Acad Sci USA, 1989; 86: 3466-3468.
- Pauling L, Herman ZS: Criteria for the validity of clinical trials of treatments of cohorts of cancer patients based on the Hardin Jones Principle. Proc Natl Acad Sci USA, 1989; 86: 6835-6837.
- Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, Ames MM: High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. New Engl J Med, 1985; 312: 137-141.
- Cameron E, Pauling L: Cancer and vitamin C: discussion of the nature, causes, prevention, and treatment of cancer, with special reference to the value of vitamin C. Menlo Park, CA. Linus Pauling Institute of Science and Medicine. 1979; 238 pages. Updated and expanded edition: Philadelphia, PA. Camino Books, Inc. 1993; 5.
- Cameron E, Campbell A: Innovation vs. quality control: an ‘Unpublishable’ clinical trial of supplemental ascorbate in incurable cancer. Med Hypoth, 1991; 36: 185-189.
- Richards E: Vitamin C and cancer: medicine or politics? New York. St. Martin’s Press. 1991.
|




 Back to 1998 4th Quarter Table of Contents
Back to 1998 4th Quarter Table of Contents
