 |

|
 |

|
 Back to 1998 2nd Quarter Table of Contents
Back to 1998 2nd Quarter Table of Contents
Abstract The roles of animal fat and sweeteners (mono and disaccharides) in acute myocardial infarction and other ischemic heart disease are examined through multicountry statistical associations between the dietary components and the heart disease mortality rates for 1986 for males and females aged 35-74 in 33 countries. For both acute myocardial infarction and other ischemic heart disease, it is found that dietary animal fat is the primary dietary macronutrient risk factor for mortality among males, with some contribution from sweeteners, while sweeteners are found to be the primary dietary macronutrient risk factor for females. Similar results are found for time-variations of coronary heart disease in Spain, 1965-1990. The likely mechanism linking sweeteners to heart disease is that it raises serum triglycerides and, hence, very low density lipoprotein levels. Similar results were reported in the 1960s, but only for males. While these results appear to be robust, others may want to reexamine these findings through other approaches. Keywords: animal fat, disaccharides, fructose, ischemic heart disease, lipoprotein, monosaccharide, myocardial infarction, sucrose, sweeteners, triglycerides. Objectives It is widely accepted that consumption of animal fat is a risk factor for coronary heart disease (CHD).1-4 A perusal of the 1960s literature indicates that there is also a link between dietary sugars and heart disease, although this fact is overlooked in the more recent literature. Yudkin5 seems to have made the first multi-country link between sugar and coronary heart disease, but could not exclude animal fat from also having a role. In the 1960s, he strengthened the case for sugar.6,7 Osancová et al.8 showed that sugar consumption correlated better than fat consumption with cardiovascular disease mortality in Czechoslovakia. Others9-11 showed that dietary sucrose increased total serum cholesterol levels, while complex carbohydrates could lower serum cholesterol.10 Sucrose was shown to be associated with increased serum cholesterol or lipoprotein compared with vegetables and legumes (0.1 for a 0.10-0.14 exchange of calories)12 or purified wheat (up to 0.14 for a 0.15 exchange of calories).13 In addition, Macdonald14 showed that women aged 54-68 years had an average 30 mg higher serum cholesterol after being fed diets containing 7.5 g/kg of body weight of sucrose compared to purified wheat starch for 25 days. Keys15 strongly attacked Yudkin’s claim that dietary sucrose was a major factor in the development of coronary heart disease, pointing out that some countries with high sugar consumption and low CHD incidence were omitted, that dietary fat and sugar are highly correlated, and that the increases in cholesterol from sucrose may not be a long-term effect. Yudkin mounted a defense in his book, Sweet and Dangerous,16 but to little avail. Reiser17 reviewed the evidence to 1982, concluding that sucrose was one of the environmental factors contributing to the high levels of blood cholesterol in developed societies. In a 1986 review, Jukes18 concluded that there was enough contradictory evidence that the sugar/heart disease link could not be established. By the mid-1980s, support waned for Yudkin’s hypothesis that dietary sucrose was a risk factor for CHD. Although some resistance to the recommendation of a low-fat, low-cholesterol diet to prevent CHD occasionally surfaced,19,20 it has been adopted.3,21 Recently, however, several papers have presented evidence suggesting that carbohydrates may be involved in the etiology of heart disease. A study of 72 premenopausal women between the ages of 25 and 44 in switching from a 0.35 fat diet to a 0.21 fat diet with 0.65 carbohydrates (half simple, half complex) and found that cholesterol levels jumped 0.15 to 0.33 depending on the LDL subclass pattern of their parents.22 Another study showed that a 0.60-carbohydrate/0.25-fat diet leads to higher plasma triacylglycerol, very-low-density-lipoprotein (VLDL) and VLDL-cholesterol than a 0.4-carbohydrate/0.45-fat diet.19 The sugar:starch ratio in each diet was 0.33:0.66. Case control study data by Knopp et al.23 shows that lowering fat from 0.35 to 0.22-0.27 while increasing carbohydrates from 0.45-0.46 to 0.52-0.60 for those with hyper-cholesterolemia lowers LDL cholesterol but raises triglycerides after 12 months. Gaziano et al. present data on the relative risk ratios for case controlled studies of triglyceride levels, showing a superlinear relationship.24 Evidence from clinical trials presented by Oliver25 indicates that increasing the intake of unsaturated fats is more likely to lead to reduced ischemic heart disease than reducing saturated fat intake. Katan26 also shows that low-fat, high-carbohydrate diets do not reduce ischemic heart disease, probably since triglycerides levels rise. Finally, a recent study shows that carbohydrates increase the risk of coronary heart disease in women compared with mono- and polyunsaturated fats, but not in comparison with saturated fat.27 No distinction was made between simple and complex carbohydrates in these studies. Thus, there is sufficient reason to suspect that sugar may, indeed, be involved in the etiology of heart disease. Methods To investigate whether sweeteners can be considered a risk factor for heart disease, the multi-country statistical approach was employed. In this approach, national disease statistics are compared with components of the national dietary consumer supply values in a multi-variate model. The components with statistically-significant associations with the disease are then examined further for links to that disease to establish their role as risk factors. While this approach has been successfully applied in the past, it has fallen out of favor in the medical research community for several reasons. One, called the ecological fallacy, is that it is hard to guarantee that some unknown confounding variable is not affecting the analysis.28 Confounding between animal fat and sugar6 played an important role in the Keys-Yudkin debate. In addition, in studies using mortality from disease, the medical care delivery system of the country can affect the statistics. Also, the national consumer dietary supply29 is not necessarily a good measure of the diet that actually pertains to those in the disease study. Finally, the approach is rather coarse in that dietary components that comprise a small fraction of larger components of the diet are generally not broken out. For example, the various fatty acids are not listed separately. In defense of the multi-country approach, it has generally yielded results which are consistent with what is or has been accepted regarding dietary risk factors for various diseases. For example, this approach has been successfully used to link low-fiber diets to appendicitis;30,31 high-fat, low-carbohydrate, high-lipid diets to diabetes mellitus;32 dietary sugar to heart disease;5 animal fat to CHD;1 and dietary fat to colon cancer33 and Alzheimer’s disease.34 Successes with the multi-country approach are often verified by congruence between epidemiologic, clinical, and laboratory research findings.28 For dietary factors, the Food Balance Sheets of the Food and Agriculture Organization (FAO)29 are used. The FAO regularly publishes a detailed summary for many components of the diet for 179 countries in three-year intervals through 1994.35 The values represent food available to the consumer, and do not take into account losses due to spoilage, wastage, etc. Thus, they overestimate the amount actually consumed. In the U.S.A., the reduction was recently estimated to be 0.25. However, it is assumed that similar reduction factors are found in other countries used in the study. Sweeteners in their tables include sugar, non-centrifugal; sugar (raw equivalent); sweeteners; and honey, with 0.95-1.00 of the calories coming from sugar. In this study, regression analyses were performed for 1986 mortality rate data for acute myocardial infarction (AMI) and other ischemic heart diseases (OIHD) for four 10-year age groupings from 35 to 74 for 33 countries36 (Appendix 1, p. 83). Countries were initially screened for life expectancy in 1970 greater than 69 years and populations over 1 million. Only Eastern European countries (Bulgaria, Hungary, Poland, Romania, and the USSR) were intentionally excluded because the high heart disease mortality rates in these countries indicate a systematic difference with respect to other countries. Dietary supply data for 1982 (averaged from 1979-1984) and 1973 (averaged from 1972-74) were also used in the analysis.29 Results The AMI mortality data for those aged 65-74 were first used in a multiple linear regression analysis with alcohol, animal fat, calories, cereals, cigarettes,37 fat, fish, fruit, life expectancy,38 starchy roots, stimulants, sweeteners, and vegetables. Only animal fat and sweeteners were found to be statistically significant (p<0.05). Even with smaller subsets of variables, these two were still the only variables which yielded significant results. However, for linear regressions, five variables were found to be statistically significant for males age 65-74: sweeteners (r2 = 0.347, p <0.001); animal fat (0.587, <0.001); fat (0.509, <0.001); calories (0.169, 0.017); and cereals ((-)0.398, <0.001), where (-) denotes an inverse association. For AMI and females age 65-74, only four variables were found to be statistically significant: sweeteners (0.486, <0.001), animal fat (0.333, <0.001), cereals (0.271, 0.002), and fat (0.259, 0.003). Four of these five variables have been mentioned in the past as having some possible association with heart disease. The model examined was that animal fat and sweeteners together comprise the primary dietary risk for AMI. The p value for cereals in multiple regressions showed it to be not statistically significant. Scatter plots of the highest linear associations for AMI are given in Table 1 (p. 98) and shown graphically in Figures 1 and 2 (p. 99). For males, dietary animal fat supply for 1973 has a significantly higher correlation for AMI mortality rates than does either animal fat for 1983 or sweeteners. Possibly the effect of animal fat builds up slowly through the years. For females, dietary sweetener supply has a much higher correlation with AMI mortality rates than does animal fat, with the results rather independent of year. Figures 3 and 4 (p. 100) present the statistical results for AMI for the four age groups considered. For males, animal fat is the primary dietary risk factor, but sweeteners seem to help, with the F value (F = t2, t = student t-test) not changing much. For females, sweeteners is the primary dietary risk factor, with animal fat contributing. The F value is nearly the same for both calculations for age 65-74, but drops to about half for the pair compared to sweeteners alone for age 35-44. The OIHD mortality data were first tested with a number of factors with similar results as for AMI. Thus, the same model, that animal fat and sweeteners are the primary dietary factors raising the risk of OIHD was adopted. The results are presented in Table 1 and Figure 5 (p. 101). First, the statistical associations for the various age groups are similar to those for AMI. The combined animal fat/sweetener association Table 1. Statistical results (r2 and p) for AMI and OIHD mortality rates (WHO)36 for 1973 animal fat and 1983 sweeteners (FAO).29 AMIMalesFemales Animal Fat Sweeteners Both Animal Fat Sweeteners Both 65-74 0.608* 0.347* 0.708* 0.333* 0.486* 0.642* 55-64 0.496* 0.256** 0.614* 0.187** 0.433* 0.520* 45-54 0.376* 0.193** 0.438* 0.048*** 0.284* 0.271** 35-44 0.061*** 0.065*** 0.092*** 0.00*** 0.103*** 0.139*** OIHD Males Females Animal Fat Sweeteners Both Animal Fat Sweeteners Both 65-74 0.573* 0.369* 0.687* 0.264** 0.572* 0.632* 55-64 0.578* 0.216** 0.609* 0.312* 0.421* 0.536* 45-54 0.513* 0.163** 0.531* 0.214** 0.209** 0.331** 35-44 0.287** 0.054*** 0.288** 0.137*** 0.349* 0.362** p values: * <0.001; ** <0.05; *** >0.05 Table 2. Statistical results for trends in coronary heart disease in Spain, 1965-1990.34 Dietary data for the year of the mortality rate data are used.35 Males Dietary component r2 F p Animal fat 0.501 7.0 0.033 Fat 0.557 8.8 0.021 Sweeteners 0.495 6.9 0.034 Animal fat + sweeteners 0.802 12.1 0.008 Females Animal fat 0.259 2.4 0.124 Fat 0.303 3.0 0.124 Sweeteners 0.623 11.6 0.011 Animal fat + sweeteners 0.730 8.1 0.020 generally decreases with age of the groups, except for females 35-44. Second, animal fat generally has a higher association than sweeteners for men. The exceptions are for males 65-74 and men 35-44 with the 1982 dietary data. For women, sweeteners are always found to be much more highly associated 98 with OIHD than animal fats, which often had inverse associations, although not statistically significant ones. Animal fats from 1973 were found to be more highly associated with OIHD in men than the 1982 values. Figure 2 (p. 79) shows linear regression data for sweeteners for 65-74-year-old females. Figure 1. Scatter plot of AMI 1986 mortality rates (MR) for males age 65-74 vs. 1973 animal fat supply according to the regression result, MR = 173.273 +5.489 x animal fat (g/day). 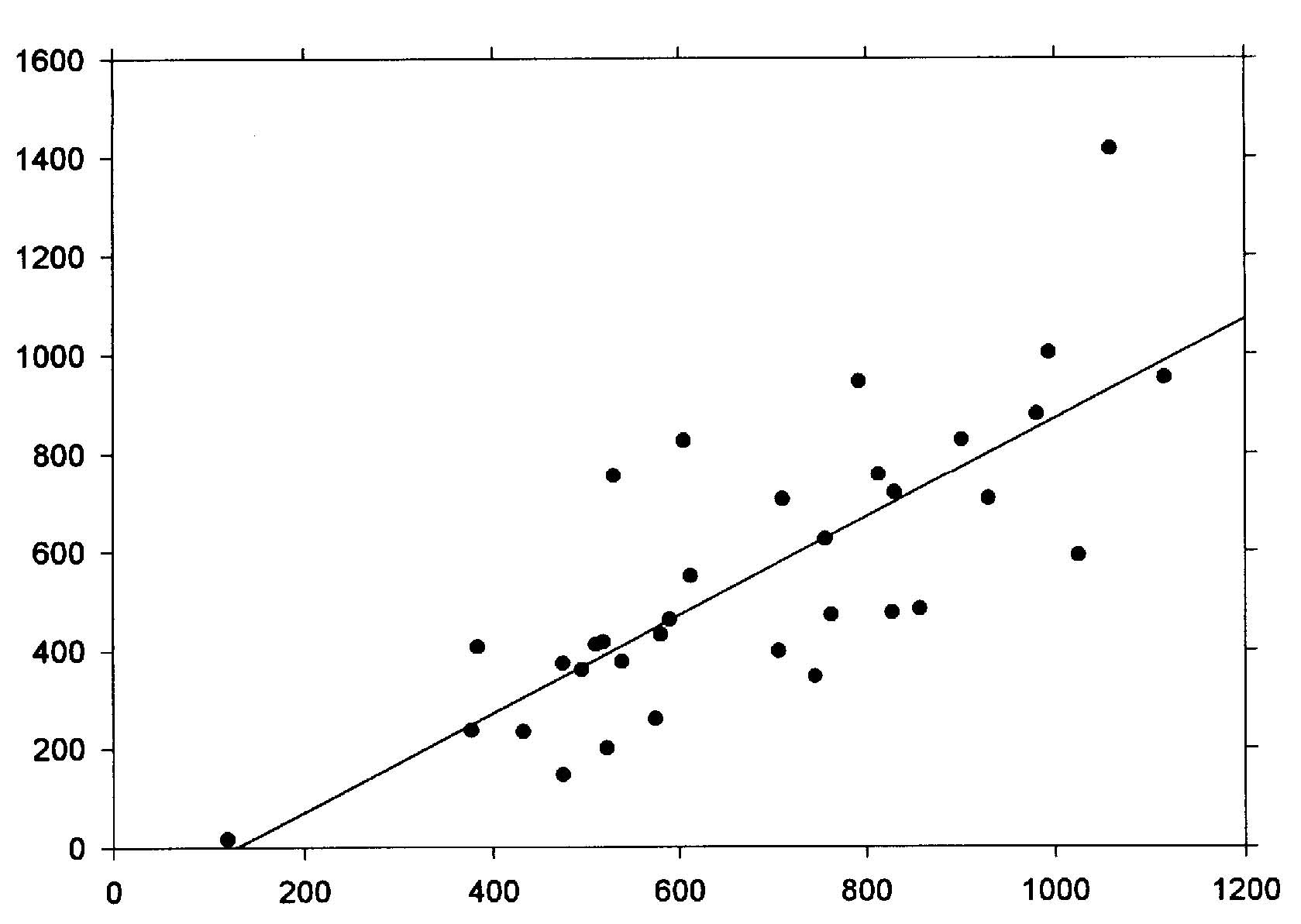 r2 = 0.608, p<0.001 0.387 X Animal fat (cal./day) + 1.188 X Sweeteners (cal./day) Figure 2. Scatter plot of AMI 1986 mortality rates for females age 65-74 vs. 1983 sweetener supply according to the regression result, MR = -40.857+ 0.704 x sweeteners (cal/day). 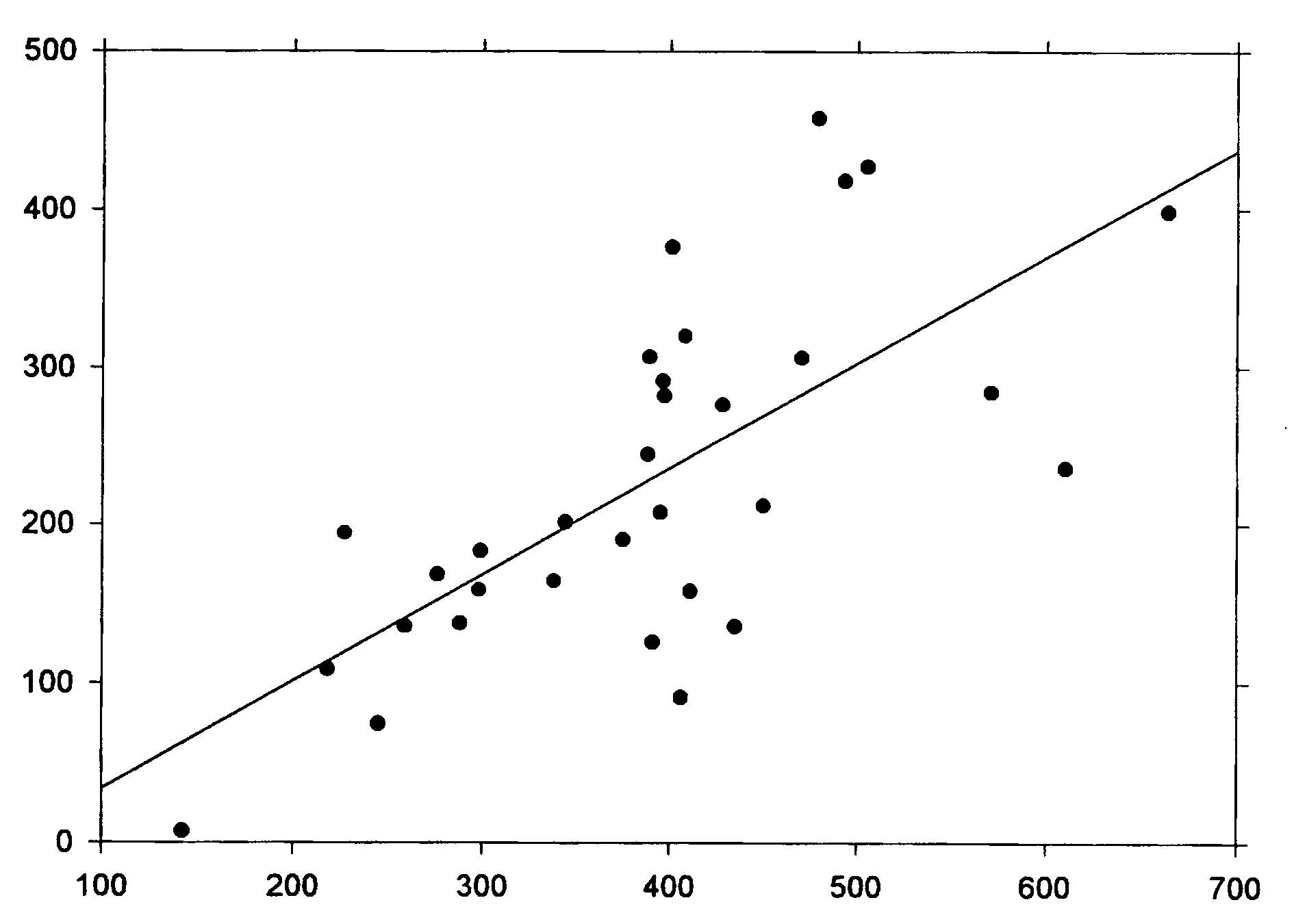 r2 = 0.486 1983 Per capita sweeteners supply (calories/day) Figure 3. Acute myocardial infarction rates (males 1986) vs. dietary supply. 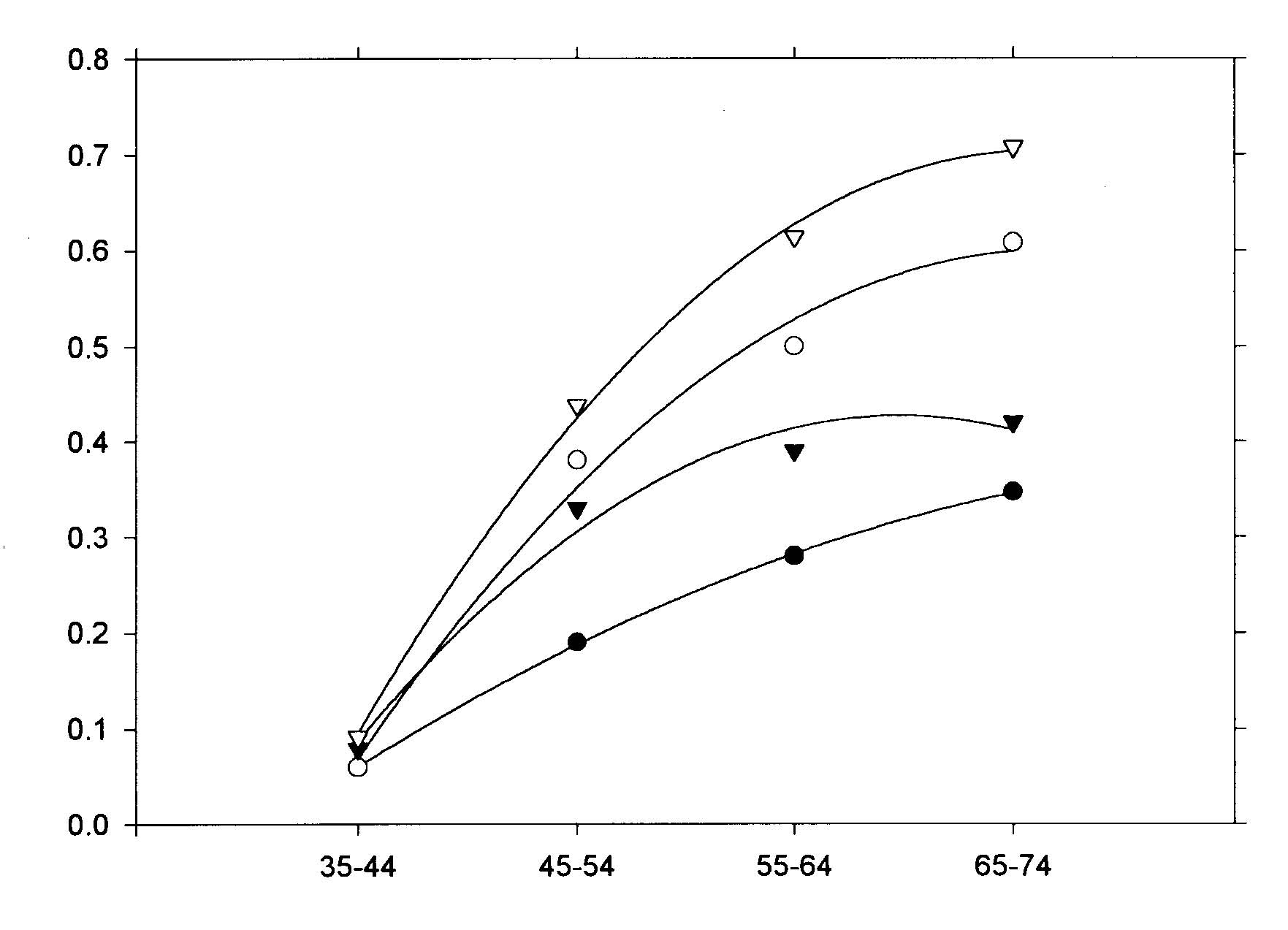 animal fat-1973 + sweeteners-1983 animal fat-1973 animal fat-1983 sweeteners-1983 Age range (years) Figure 4. Acute myocardial infarction rates (females 1986) vs. dietary supply. 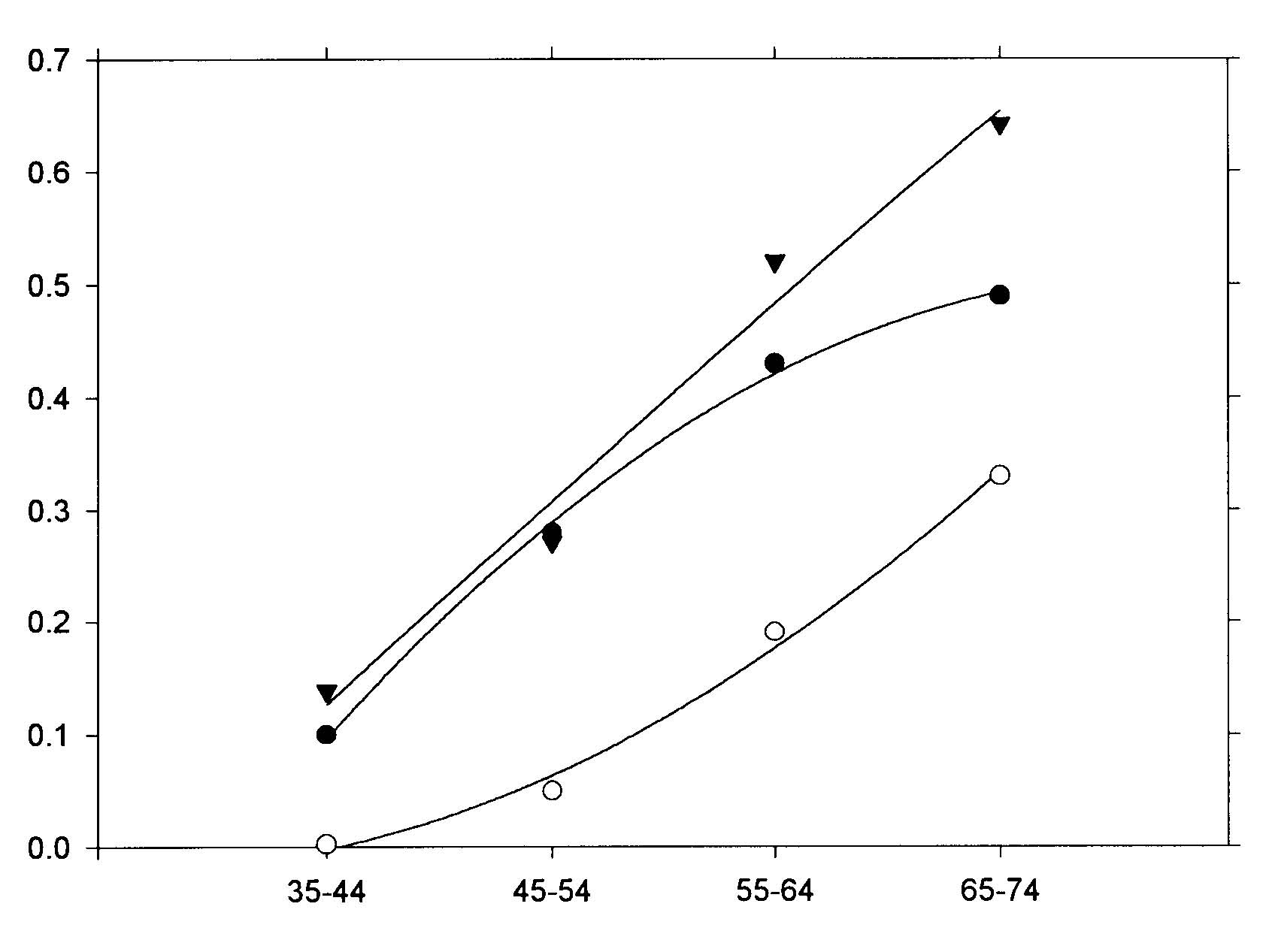 Age range (years) 100 Figure 5. Scatter plot of OIHD 1986 mortality rates for females age 65-74 vs. 1983 sweeteners, according to the regression result, MR = -60.946 + 0.414 x sweeteners (cal/day). 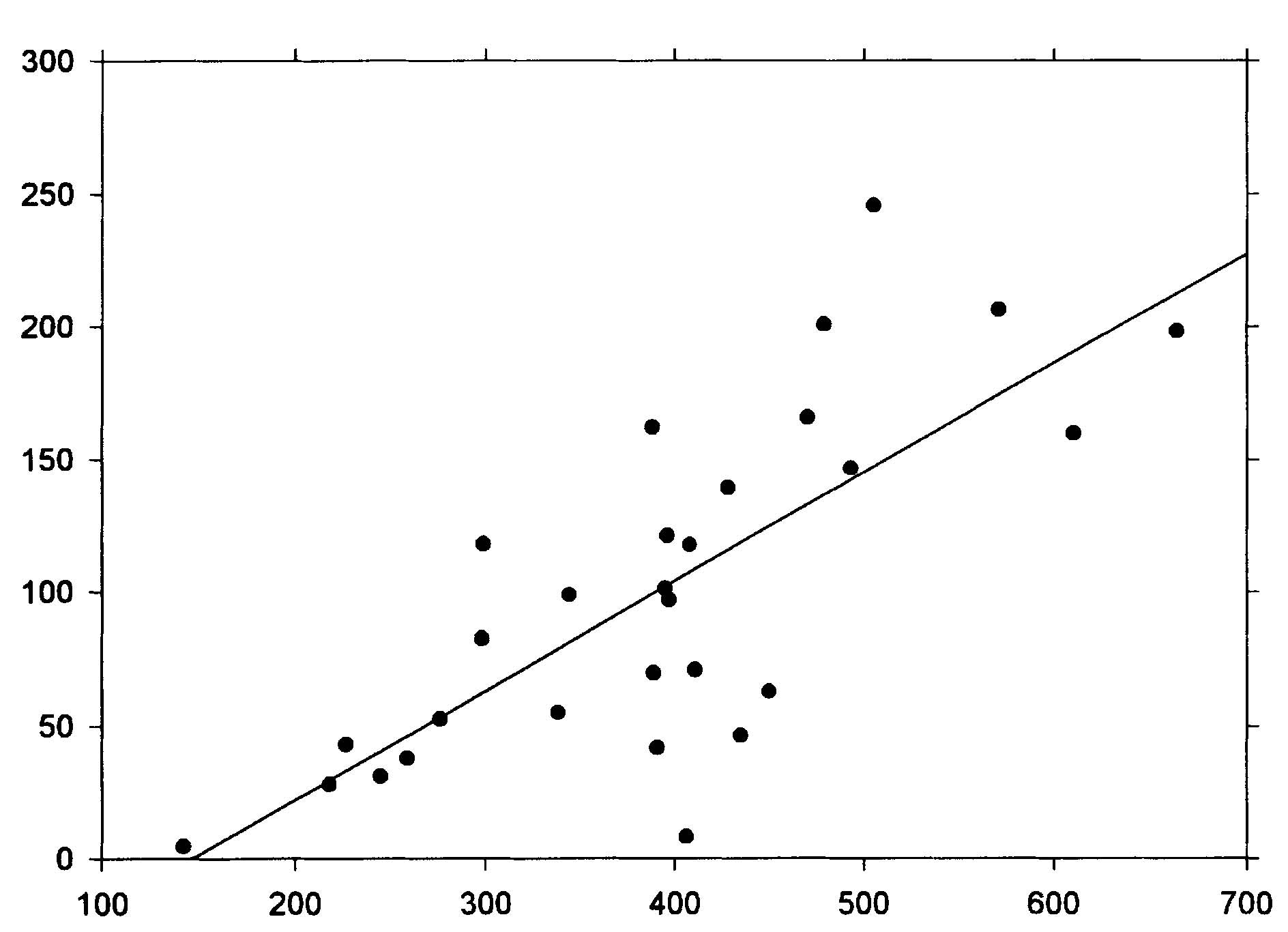 r2 = 0.572 1983 Per capita sweeteners supply (calories/day) Data from Spain for trends of CHD mortality39 can be used as a further check. Regression analyses were run for the current year dietary data as well as for two, four and six years prior to the mortality data. The results are shown in Table 2 (p. 78) for current year dietary data. The results support the findings of the geographical distributions for females. However, for males, only the current year data show a strong association with animal fat. For other years, sweeteners have a much higher r2, even higher than for females for four and six years prior diets. The authors of the study did not examine sweeteners as a possible factor. Discussion Supporting data for the role of sweeteners in the etiology of heart disease also comes from the island of St. Helena (15.57°S, 5.42° W). As reported Shine et al.40 and discussed by Hoffer,41 people there exercise a lot, don’t smoke much, and have low fat intake, but still have a very high incidence of heart disease. Their sugar intake had increased to 125 pounds per person per year. Thus, these analyses suggest that epidemiologic data support the finding of the 1960s that dietary sucrose is a risk factor for heart disease. Returning to the studies of Keys1,15 and Yudkin,5-7 we see that they did not use sophisticated statistical analyses techniques, such as multivariate analyses, so were unable to examine carefully the separate roles of animal fats and sweeteners. In addition, they did not study the mortality rates of females, where the sugar effect is larger. While associations are useful in trying to make the link between diet and disease, the case is strengthened considerably when mechanisms can be found, such as a step-by-step linkage between dietary components and disease. The proposed mechanism linking sugar to heart disease is the synthesis of serum triacylglycerols (triglycerides or TG) by the liver, followed by increased production of very low density lipoprotein (VLDL). Sucrose is a disaccharide which splits into glucose and fructose in the first metabolic step.42 Fructose proceeds through several steps in the liver before generally being synthesized into phosphoglycerides and TG. Triglycerides, in turn, are incorporated into plasma lipoprotein (colloquially, cholesterol): VLDL is 50-60% TG, 20% cholesterol and cholesterol esters (CCE), 15-20% phospholipids (PPL), and 5-10% protein. LDL, derived from VLDL, is 8% TG, 22-40% CCE, 22-30% PPL, and 20-22% protein; and high density lipoprotein (HDL), formed in both the liver and intestines, is 5-8% TG, 16-25% CCE, 24-30% PPL, and 40-52% pro-tein.42,43 Thus, the more fructose in the diet, the more production of TG, leading to more VLDL and LDL. Both TG and VLDL are considered to be high risk factors for IHD.24 Syndrome X was defined in 198844 as a cluster of abnormalities occurring in nondiabetic persons that increase the risk of coronary heart disease.45 The components of syndrome X were originally identified as including some degree of glucose intolerance, a high TG and low HDL concentration and an increase in blood pressure. Recently, atherogenesis has been added to the syndrome.45 The results of this paper identify simple sugars as the primary cause of serum TG and VLDL. This conclusion is strongly supported by the results of Ref. 10, which show that for 16 Asian and South American countries, primarily from data on the armed forces, the cholesterol levels are directly proportional to the fraction of calories from simple sugars (mono and disaccharides) (r=0.81) and inversely related to the fraction of complex sugars (polysaccharides) (r=-0.72), with a very weak dependence on the fraction of calories from fat (r=0.25). Hudgins et al.46 provide recent support for this conclusion through clinical studies. Barker et al.47 also point out that LDL-cholesterol levels are not necessarily affected by dietary fat. Based on the principles of syndrome X, the national dietary data were used to see whether carbohydrates other than the simple sugars play a role in the etiology of AMI. For this analysis, the carbohydrate portion of cereals and starchy roots were included in a multiple linear regression with animal fat and sugars for those aged 65-74. The p values for the carbohydrates from cereals and roots were found to be 0.14 and 0.63, respectively, for males and0.46 and 0.41 for females, with cereal carbohydrates inversely associated with AMI (i.e., statistically insignificant). This result gives further support to the finding that fructose is very important in the etiology of heart disease, and does not support glucose as having a major role, since glucose is a major component of starch.42 Sugars in the diet also lead to insulin resistance due to excesses of glucose.48 Glucose is found in fruits, sweet corn, honey, sucrose, lactose, maltose, and many of the complex carbohydrates.42 While glucose can also metabolize to become TG,43 evidently this is less common than for fructose. An important question raised by the analysis in this paper is why the associations between sweeteners and heart disease are higher for females than for males. It could be that women eat more sugar than men do. Barker et al.47 showed that Type A women in the UK had weak positive correlations with sugar and alcohol intake, while men had weak positive correlations with protein and fat intake (Type A personalities are more prone to stress and heart disease). It could also be that the female physiology is better able to assimilate animal fat. For example, estrogen replacement therapy has been shown to reduce the risk of AMI in postmenopausal women.49 Women generally have lower cholesterol levels than men until age 55, at which time their cholesterol levels surpass those of men the same age. Jeppesen et al.20 showed in case control studies that a 0.60-carbohydrate diet resulted in a higher risk for IHD for postmenopausal women than did a 0.40-carbohydrate diet since it resulted in a decrease in high density lipoprotein (HDL) cholesterol and an increase in fasting plasma triglyceride concentrations. The fact that heart disease mortality rates increase with age seems to be consistent with increases in serum lipoproteins with age. Miller50 attributes the increases to reduced catabolis by receptors in hepatic and extrahepatic tissues, and partly by receptor independent mechanism as people age. Conclusions The findings in this paper strongly suggest that excess dietary animal fats and sweeteners are both major risk factors for AMI and OIHD, especially as people age. The role of sweeteners has not been investigated very thoroughly since Keys’ 1971 paper15 and 1975 paper,51 although several recent papers have pointed out that high-carbohydrate, low-fat diets do not reduce heart disease rates as much as would be expected if fat were the primary dietary risk factor. Given that low-complexity carbohydrates seem to increase the risk of heart disease while complex carbohydrates seem to reduce the risk,10 investigations of carbohydrates ranked according to complexity should be investigated for their role in the etiology of heart disease, much as the various fatty acids are now.27 In addition, differences in male/female physiology or dietary preferences regarding animal fat and sweeteners should be investigated. Acknowledgements The author thanks the staffs and supporting organizations of the Moorman Memorial Library of the Eastern Virginia Medical School (Norfolk, Va.), the Health Sciences Library of the Riverside School of Professional Nursing (Newport News, Va.) and the Stanford Medical School’s Lane Medical Library for the use of their facilities. Appendix 1. Countries included in the AMI and OIHD mortality studies: Argentina, Australia, Austria, Belgium, Canada, Chile, Costa Rica, Cuba, Ecuador, England/ Wales, France, Germany, Greece, Hong Kong, Ireland, Italy, Japan, Korea, Kuwait, Mauritius, Mexico, Netherlands, New Zealand, Norway, Panama, Paraguay, Portugal, Singapore, Spain, Sri Lanka, Uruguay, USA, Venezuela. Denmark, Finland, Sweden, and Switzerland were not included because the mortality data are not available for AMI and OIHD separately. References
Clin Nutr, 1966; 18: 86-90.
betes mellitus. Diabetes Care, 1935; 1: 117-48.
|

This website is managed by Riordan Clinic
A Non-profit 501(c)(3) Medical, Research and Educational Organization
3100 North Hillside Avenue, Wichita, KS 67219 USA
Phone: 316-682-3100; Fax: 316-682-5054
© (Riordan Clinic) 2004 - 2024c
Information on Orthomolecular.org is provided for educational purposes only. It is not intended as medical advice.
Consult your orthomolecular health care professional for individual guidance on specific health problems.