The Nutritional Relationships of Iron
David L. Watts, Ph.D., F.A.C.E.P.1
Iron deficiency anemia has been considered the major nutritional problem throughout the world. However, it is now known that iron is involved, in many metabolic processes, particularly enzymes, and that many clinical manifestations (other than anemia) can develop as a result of iron deficiency. Iron is present in every cell and is involved in electron transport via cytochromes in the Krebs cycle, oxidases, and oxygenases.1
Body Content and Requirements
The iron content of the body is approximately 25 mg per kg in adult women and 35 mg per kg in men. The RDA for iron is 10-15 mg for infants and children, 10 mg in adult males, 18 mg for teenagers and adult women, 45 mg during pregnancy and lactation.
Iron Assessment, Blood and Tissue
Routine serum tests for iron do not reflect intake of iron but are used to determine body stores, deficiencies, or overload. Alpers, et al, have indicated the inherent instability of serum iron, which fluctuates as much as 30% in the same person daily.2 Statland and Winkel reported a considerable variation of serum iron levels in blood samples taken from the same patient at the same time each day.3 Due to a normal diurnal variation, morning values can be as much as 30% higher than evening values. This rhythm is reversed in night workers.4 Sleep deprivation can produce as much as a 50% reduction in serum iron levels. 5 Serum levels of iron are also known to fluctuate with the menstrual cycle and oral contraceptive (OCA) use. Iron levels decrease just prior to menstruation and elevate during OCA use.
Due to the normal and induced variations in serum iron concentrations, single
1. Trace Elements, Inc., P.O. Box 514, Addison, TX 75001.
iron determinations should be interpreted cautiously when assessing iron deficiency or iron overloads.
Tissue mineral analysis (TMA) has a unique advantage over serum in that it has the potential for detecting a tendency toward iron imbalance, either a deficiency or overload, before serum changes can be detected. TMA studies are not subject to the normal diurnal variations found in serum. Since iron deficiency can exist without anemia (sideropenia) and has been reported to be 2 to 3 times more predominant that iron deficiency anemia6 7, TMA may serve as an appropriate screening tool for iron status.
TMA iron levels should also be interpreted carefully. Sample variations and possible exogenous contamination should be considered when interpreting TMA results.8 Trace Elements, Inc., has established the ideal tissue iron level at 2.8 mg%. However, tissue iron status should not be interpreted by the iron level alone, but more importantly in relationship with other minerals and, of course, in conjunction with serum test results and clinical findings. Significant ratios include calcium to iron (Ca/Fe), iron to copper (Fe/Cu), iron to lead (Fe/Pb), and iron to mercury (Fe/Hg). The ideal ratios are Ca/Fe = 15, Fe/Cu =1.12, Fe/Pb = >5.6, and Fe/Hg = >28. Significant changes in these ratios (elevated Ca/Fe, lowered Fe/Cu, Fe/Pb, and Fe/Hg) can indicate the tendency toward iron deficiency, since each of the metals is known to interfere with iron absorption or the metabolic processes that require iron. Iron overload can also be assessed by using the tissue iron level in conjunction with copper and calcium (low Ca/Fe and elevated Fe/Cu).
Other routine serum indications that may be used in conjunction with TMA studies include total iron-binding capacity (TIBC), percentage saturation of transferrin, ferritin, hematocrit (HCT), mean corpuscular volume (MCV), and mean cor-
110
Nutritional Relationships of Iron
puscular hemoglobin (MCH). These serum changes usually only manifest when iron storage is becoming depleted. They are also subject to a number of variations such as altitude, physiological status, age, and sex. Further discussion of these and other tests are well described by Sauberlich, et al.9
Conditions Associated with Iron Imbalance
Prasad has reported that the recorded therapeutic use of iron dates back to 500 B.C.10 Iron was used to treat a condition known as chlorosis, which manifested in pallor of the skin, heaviness of the body and extremities, edema, dyspnea, palpitations, headaches, rapid pulse, prolonged sleep, cessation of menses, and aversion to eating meats. With the present knowledge of the biochemical functions of iron, this list can be extended to include anemia and non-iron deficiency anemia manifestations (sideropenia), which include emotional changes, immunodeficiency, endocrine, and other physical disorders.
Mental Effects of Iron Deficiency
Early studies have reported a correlation between hemoglobin levels and intellectual performance in teenagers.11 Other studies have confirmed these findings in adults as well as children.12 13 Further studies have concluded that iron deficiency occurring with and without anemia can lead to defects in attention and cognitive functions,14 minimal brain dysfunction, and hyperactivity.15 Disturbances in mental functions have been attributed to a number of defects in the metabolic pathways that require iron such as a simple reduction in oxygenation of brain tissues due to low hemoglobin. Iron is required for DNA synthesis in mitosis; therefore, a deficiency may lead to impaired neuronal development. Iron is also required in enzymes involved in neurotransmission. The iron dependent enzyme monoamine oxidase (MAO), is reduced in the presence of iron deficiency. A reduction in MAO activity leads to an increase of other neurotransmitters such as norepinephrine, dopamine, and serotonin. Studies have reported that in iron deficient children the urinary excretion of norepinephrine is increased.16
Through TMA studies, we have often observed that patients who show excessive tissue iron accumulation, or low Ca/Fe and elevated Fe/Cu ratios, are more intellectually oriented, indicating left hemisphere brain dominance. Decreased left hemisphere activity has been confirmed in iron deficiency patients through EEG studies.17 18 We have also observed that too much tissue iron accumulation leads to aggressive behaviour, hostility, and hyperactivity. It can, therefore, be tentatively concluded that elevated TMA iron levels, low Ca/Fe or high Fe/Cu indicate left hemisphere dominance (intellectually oriented), and low tissue iron, or high Ca/Fe and low Fe/Cu indicate right hemisphere dominance (emotionally oriented).
Other Physical and Metabolic Effects of Iron Imbalance
A number of physical effects are associated with iron deficiency. The symptoms of chlorosis were described earlier. Other symptoms include intracranial hypertension resulting in chronic dull headaches, enlargement of the spleen and heart, glossitis, angular stomatitis, and koilonychia (concave or spoonshaped nails).19 When anemia is present, the sclera of the eye may become pearly white or blue. The nails become brittle and ridged longitudinally.20
Pica
Pica, a term used to describe an abnormal or unusual craving for foods or other substances, is associated with iron deficiency. This condition is found most often in children and women.21 The most common form of pica is geophagia, which is the ingestion of clay or dirt. Paper pica has been reported in individuals suffering from mercury toxicity in conjunction with iron deficiency. An example, involves a woman who constantly ate paper in the form of paperback novels, tissue paper and boxes, cigarette and candy wrappers. She was found to have mercury toxicity in both the blood and hair as well as iron deficiency anemia. Mercury is used to preserve paper products and acts as a fungicide. Oral iron therapy eliminated her desire for eating and chewing paper. Pagophagia, the compulsive eating of ice
111
Journal of Orthomolecular Medicine Vol. 3. No. 3. 1988
common in adults, is controlled with iron therapy.22 The ingestion of or craving for laundry starch (amylophagia) is a form of pica that frequently occurs in women, particularly when pregnant, and is another sign of iron deficiency.23 Pica is generally considered a phenomenon that occurs in underdeveloped countries or in poor rural areas, but it is not uncommon in the general population. It should also be noted that pica can occur in the presence of adequate or excessive iron stores. An increase in tissue retention of iron without the ability for it to be reutilized in the hemopoietic process produces the same effect as iron deficiency.
Endocrine Effects of Iron Imbalance
In 1938 Cecil described the relationship between hypothyroidism and iron deficiency anemia. He stated that both thyroid extract and iron therapy are indicated for iron deficiency anemia.24 More recently researchers have found that iron deficiency can impair thyroid function and that iron status may reflect thyroid activity.25 Lehmann, et al, recently reported that the conversion of L-phenylalanine to L-tyro-sine was reduced over 50% in iron deficient subjects.26 Since there is a reciprocal relationship between the thyroid and adrenal glands,27 28 29 it can be speculated that iron status may also indicate adrenal activity. TMA studies have revealed that subclinical hyperparathyroidism may contribute to iron deficiency. This would be expected due to the increased absorption of calcium influence by the parathyroid, thereby decreasing iron absorption.
Dysphagia
A condition known as the Plummer-Vinson Syndrome (PVS) is associated with iron deficiency and characterized by esophageal stricture causing difficulty in swallowing. Vinson originally described these cases as hysterical dysphagia. PVS is known to respond to iron therapy.30 It is possible that a decrease in thyroid function may have the effect of exacerbating PVS. Difficulty in swallowing or a frequent need to clear the throat is a classical sign of hypothyroidism or enlargement. As discussed previously, iron deficiency is related to low thyroid activity. Perhaps those
patients who complain of difficulty in swallowing, especially pills, should have their thyroid and iron status evaluated.
Infections
During an infectious condition (bacterial), iron is shifted into storage compartments — the liver, spleen, and bone marrow. This is a protective mechanism rendering the iron unavailable to the bacteria, which requires iron for its growth.31 32 Iron also becomes unavailable for incorporation into red blood cells, resulting in an infectious anemia that does not respond to iron therapy until the infection is controlled. Chronic infections (bacterial) are indicated on TMA studies by an elevation in the Fe/Cu ratio, usually greater than 2 to 1. Other conditions in which iron reutilization is impaired include rheumatoid arthritis and some types of malignancies. The above conditions result in iron deficiency anemia, even though tissue concentrations of iron are more than adequate. Iron deficiency is also a predisposing factor in infections due to a decrease in humoral immunity.33 The decreased immunity is probably due to other accompanying nutritional deficiencies. Low iron and hemoglobin levels have been found to be associated with vitamin A deficiency.34
Iron deficiency is also found in individuals suffering from chronic candidiasis and recurring herpes viral infections.35
Factors Contributing to Iron Deficiency
Iron deficiency can be caused by many factors: increased physiological requirements (such as during pregnancy and growth), excessive blood loss, low iron intake, poor absorption, dietary antagonists, infections, parasites, poor nutrition, and vitamin and mineral deficiencies.
Sources and Absorption
Heme iron (iron attached to a heme molecule) is the most available form of iron for absorption and is found in animal protein. Even though iron from vegetables is not as readily absorbed when consumed alone, absorption is enhanced in the presence of animal proteins. Dairy products such as milk and cheeses can reduce iron absorption by as much as 80%.36 Tannin
112
Nutritional Relationships of Iron
contained in teas also decreases iron absorption.
Iron requires an acid medium for com-plexing with proteins in the stomach for later release and absorption in the small intestine. A deficiency of hydrochloric acid markedly inhibits iron absorption. In an alkaline medium, iron forms insoluble complexes and becomes unavailable for absorption.
Minerals Antagonistic To Iron Absorption
Figure 1 indicates the minerals that are antagonistic to iron.37 38 39 40 41 42 Excessive intake of any one or combination of these trace elements can contribute to siderope-nia and iron deficiency anemia or exacerbate an existing deficiency. Antagonism of iron by these minerals occurs either by inhibiting absorption, compartmental displacement, or interfering with cellular iron enzymes. Iron therapy can also be used in antagonizing the effects of toxic metal accumulation.
A deficiency of the synergistic trace elements can also lead to iron deficiency. The synergistic minerals include the following:
Minerals Synergistic to Iron
Copper (Cu) Chromium (Cr)
Sodium (Na) Nickel (Ni)
Selenium (Se) Potassium (K)
Manganese (Mn) Phosphorus (P)
Some elements are listed as both antagonistic and synergistic because of a dual relationship of some elements with iron. As an example, copper is considered synergistic due to its requirement in ferroxidase activity. However, excessive intake of copper competes with iron for absorption.
Vitamins Antagonistic To Iron
The vitamins that are considered antagonistic to iron are shown in figure 2. The mechanism of antagonism may be direct or indirect. As an example, vitamin D, by enhancing the absorption of calcium, can lead to poor iron absorption. Cobalt, which is an integral part of vitamin B12, competes with iron and vice-versa on an absorptive level.43
The antagonistic effects of iron and vitamin E occur on a cellular level, either through increased metabolic demands of iron produced by vitamin E or by free radical production caused by excess tissue iron accumulation. Excess tissue iron levels contribute to lipid peroxidation,44 thereby increasing the antioxidant requirement for vitamin E.45
Rarely does a single mineral deficiency develop without other nutritional deficiencies. A deficiency of synergistic vitamins may also be found with iron deficiency. Vitamins that are considered synergistic to iron are listed below.
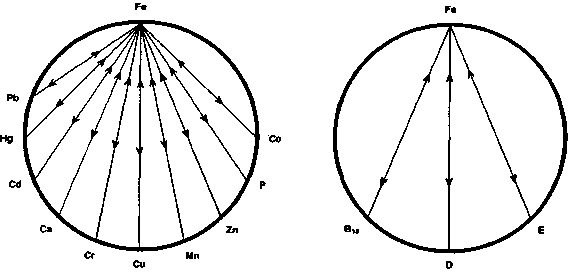
Figure 1.
Figure 2.
113
Journal of Orthomolecular Medicine Vol. 3, No. 3, 1988
Vitamins Synergistic To Iron
Vitamin E Vitamin B6
Vitamin B1 Vitamin B12
Vitamin B2 Vitamin C
Vitamin B3 Vitamin A
A deficiency of one or combination of the synergistic vitamins can both lead to iron deficiency and may also be required to correct it.
Iron Overload
Acute iron toxicity is the second most common cause of accidental poisoning in small children as a result of consuming large quantities of iron supplements.46
Fairbanks describes two types of iron overload. Hemosiderosis is described as a general increase in tissue iron without fibrotic changes. Hemochromatosis is more serious and describes tissue iron accumulation with fibrotic changes occurring in the liver, spleen, and pancreas.47 Hemochromatosis and hemosiderosis develop as a result of such metabolic disturbances as hemolytic anemias, liver disease, multiple transfusions, and the ingestion of excess amounts of iron over a long period of time. Idiopathic hemochromatosis is apparently a hereditary disorder characterized by uncontrolled iron absorption. Iron overload affects men more commonly than women.
Factors Contributing To Iron Overload
The most well-known incidence of dietary iron overload has been reported in the Bantu Africans who consume large quantities of beer brewed in iron pots. The acidity of the beer leaches large amounts of iron from the pots resulting in daily intakes of iron of over 200 milligrams.48 Cooking in iron skillets and pots can increase the iron content of foods prepared in them by as much as 3 to 400 percent.
Generally, excessive alcohol intake increases iron absorption, thereby contributing to liver cirrhosis commonly seen in alcoholics. The iron content of the liver in patients with cirrhosis can be several times higher than normal. The iron content of some alcoholic beverages is high, particularly in red wines and foreign beers.
Water is often a source of iron in regions with high iron soils. Iron water pipes are
also a source of iron, especially if the water being directed through them is acidic.49
Herbs can contribute to high iron intake. Our analysis of some of the more common herbs has found the following significantly high in iron (above 50 mg%): Peppermint Leaves, Comfrey Root, Black Chohosh Root, Chickweed, Goldenseal Root.
As with other trace elements, iron absorption and retention may be affected by individual metabolic characteristics. As an example, iron absorption is increased in individuals who have a deficiency of pancreatic enzyme excretion.50 Supplementation with pancreatic enzymes is known to decrease excessive iron absorption. Copper deficiency decreases iron elimination and therefore can contribute to increased tissue burdens.51 In the presence of a copper deficiency, excessive vitamin C intake can contribute to iron overload due to the ascorbic acid-copper antagonism, and the ascorbic acid-iron synergism. Any factor that antagonizes copper retention can potentially contribute to iron overload.
Conditions Associated With Iron Overload
In hemochromatosis, excess iron accumulation is known to deposit extensively throughout the body as hemosiderin. Most notably involved are the liver, heart, pancreas, joints, lymph nodes, and skin. Iron deposits have also been found in the anterior pituitary, thyroid, adrenal cortex, and brain.
Conditions commonly associated with excessive tissue iron accumulation observed through TMA studies include the following: Cirrhosis, Diabetes, Rheumatoid Arthritis, Allergies (Histamine), Migraines, Hypertension, Hyperactivity, Hepatitis (Bacterial), Chronic Infections (Bacterial), Emotional Disturbance.
Dexter, et al, reported their findings of autopsied brain tissue from individuals who died with Parkinson's Disease and a control group who had no history of neurological disease before death. Their results revealed a 35% increase of iron in the substantia nigra of the parkinsonian brain as compared to the controls. The copper was reduced by 34%.53 They found that lipid peroxidation was increased in the parkinsonian nigral tissue, adversely
114
Nutritional Relationships of Iron
affecting dopamine-containing cells.54 Since excess iron promotes lipid peroxidation and copper inhibits it, an imbalance of Fe/Cu may play a role not only in Parkinson's Disease but other neurological diseases as well. Excessive tissue iron or elevated Fe/Cu ratios have often been noted on TMA studies of patients with Amyotrophic Lateral Sclerosis (ALS). The future may reveal that conditions such as ALS, Huntington's Chorea, and others are conditions of free radical pathology, contributed to by excess tissue iron accumulation, copper deficiency, and other factors disturbing the balance of these trace elements.
Our observations indicate that excess tissue iron levels in patients experiencing hostility and aggression may be due to iron accumulation within the amygdyla, an aggressive center. Stimulation of the amygdyla produces aggressive behaviour, which could be mediated by iron-induced lipid peroxidation.
Conclusion
This has been a brief overview of iron and its involvement in metabolic processes. Since iron deficiency without anemia is more prevalent than iron deficiency with anemia, TMA may serve as a useful tool in evaluating individual iron status. As with other important trace elements, iron assessment should be evaluated in conjunction with its nutritional co-factors, either synergists or antagonists.
References
1. Vitale J J: Impact of nutrition on immune function. Advances in Human Clinical Nutrition. Vitale, J.J., Broitman, S.A., Eds. John Wright, Inc. Boston, 1982.
2. Alpers DH, Clouse RE, Stenson WF: Manual of Nutritional Therapeutics. Little Brown and Co., Boston, 1983.
3. Statland BE, Winkel P: Relationship of day-to-day variation of serum iron concentrations to iron binding capacity in healthy young women, Am. J. Clin. Pathol. 67, 1977.
4. Sinnah R, Doggart JR, Neill DW: Diurnal variations of serum iron in normal subjects and in patients with haemochromatosis. Br. J.Haematol., 17, 1969.
5. Kuhn E, Brodan V, Brodannova M, Fried-mann B: Influence of sleep deprivation on iron metabolism. Nature, 1967.
6. Fielding J, O'Shaughnessy MC, Brunstrom GM: Iron deficiency without anaemia. Lancet, 2, 1965.
7. Heinrich HC: Iron deficiency without anemia. Lancet, ii, 1968.
8. Watts DL, Heise TN: Balancing Body Chemistry. T.E.I., Savannah, Ga. 1987.
9. Sauberlich HE, Skala JH, Dowdy RP: Laboratory Tests for the Assessment of Nutritional Status. CRC Press, Inc., FL, 1974.
10. Prasad AS: Trace Elements and Iron in Human Metabolism. Plenum Pub., N.Y., 1978.
11. Webb TE, Oski FA: Iron deficiency anemia and scholastic achievement in young adolescents. /. Ped., 82, 1973.
12. Oskie FA, Honig AM: The effects of therapy on the development scores of iron-deficient infants. /. Ped., 92, 1978.
13. Tucker DM, Sandstead HH, Pendland JE, Dawson LL, Milne DB: Iron status and brain function: serum ferritin levels associated with asymmetries of cortical electro-physiology and cognitive performance. Am. ]. Clin. Nutr., 39, 1984.
14. Pollitt E, Saco-Pollitt C, Leibel RL, Viteri FE: Iron deficiency and behavorial development in infants and preschool children. Am. J. Clin. Nutr., 43, 1986.
15.Cantwell RJ: The long term neurological sequelae of anemia in infancy. Ped. Res., 8, 1974.
16. Voorhess ML, Stuart MJ, Stockman JA, Oski FA: Iron deficiency anemia and increased urinary norepinephrine excretion. /. Ped., 86, 1975.
17. Glick SD, Weaver LM, Meibach RC: Lateralization of reward in rats: Differences in reinforcing thresholds. Science, 207, 1980.
18. Tucker DM, Swenson RA, Sandstead HH: Neuropsychological effects of iron deficiency. Neurobiology of the Trace Elements Vol. I. Dreosti, I.E., Smith, RM. Eds. Humana Press, N.Y., 1983.
19. Lindenbaum J: The hematopoietic system. Nutrition Metabolic and Clinical Applications. Hodges, R.E. Ed. Plenum Press, N.Y., 1979.
20. Holvey DN, Ed: The Merck Manual. Merck and Co., N.Y., 1972.
21.Gutelius MF, Millican FK, Layman EM, Cohen GJ, Dublin CC: Nutritional studies of children with pica II. Treatment of pica with iron given intramuscularly. Ped., 29, 1962.
22. Reynolds RD, Binder HJ, Miller MB, Chang WWY, Horan S: Pagophagia and iron deficiency anemia. Ann. Intern. Med., 69, 1968.
23. Roselle HA: Association of laundry starch and clay ingestion with anemia in New York City. Arch. Int. Med., 125, 1970.
115
Journal of Orthomolecular Medicine Vol. 3, No. 3, 1988
24. Cecil RL: A Textbook of Medicine. Saunders Co., Phil. 1938.
25. Dillman E, Gale C, Green W, Johnson DG, Mackler B, Finch C: Hypothermia in iron deficiency due to altered triiodothyronine metabolism. Am. J. Physiol., 239, 1980.
26. Lehmann WD, Heinrich HC: Impaired phenylalanine-tyrosine conversion in patients with iron-deficiency anemia studied by a L-(2H5) phenylalanine-loading test. Am. J. Clin. Nutr., 44, 1986.
27. Guyton AC: Textbook of Medical Physiology 4th Ed. Saunders Pub., Phil., 1971.
28. Pottenger FM: Symptoms of Visceral Disease. Mosby Co., St. Louis, 1930.
29. Brown JHU: Integration and Coordination of Metabolic Processes. A Systems Approach to Endocrinology. Van Nostrand Reinhold Co., N.Y., 1978.
30. Fairbanks VF, Fahey JL, Beutler E: Clinical Disorders of Iron Metabolism, 2nd Ed. Grune andStratton, N.Y., 1971.
31. Weinberg ED: Iron and susceptibility to infectious disease. Science, 184, 1974.
32. Beisel WR, Pekarek RS, Wannemacher RW: The impact of infectious disease on trace-element metabolism on the host. Trace Element Metabolism in Animals, Vol. 2. Hoekstra, W.G., Suttie, J. W., Ganther, H.E., Eds. Univ. Park Press, Baltimore, 1974.
33. MacDougall LG, Anderson R, Katz J: The immune response in iron deficient children: impaired cellular defense mechanisms with altered humoral components. /. Ped., 86, 1975.
34. Bozorgmehr B, Gahemi M: International Symposium on Clinical Nutrition and post graduate course. San Diego, Ca. 1987. Am. J. Clin. Nutr. 46, 1987.
35. Chandra RK, Newberne PM: Nutrition, Immunity, and Infection. Mechanisms of Interactions. Plenum Press, N.Y., 1977.
36. Wilson ED, Fisher KH, Garcia PA: Principles of Nutrition. John Wiley and Sons, N.Y., 1979.
37. Settlemire CT, Matrone G: In vivo effect of zinc on iron turnover in rats and life span of erythrocyte. /. Nutr., 92, 1967.
38. Prasad AS: Trace Elements and Iron in Human Metabolism. Plenum Pub., N.Y., 1978.
39. Flanagan PR, McLellan JS, Haist J, Cherian MG, Chamberlain MJ, Valberg LS: Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterol., 74, 1978.
40. Pollack S, George JN, Reba RC, Kaufman RM, Crosby WH: The absorption of nonfer-rous metals in iron deficiency. /. Clin. Invest., 44, 1965.
41. DaviesIJT: The Clinical Significance of the Essential Biological Metals. Charles Thomas Pub., 111., 1972.
42. Kirchgessner M, Schwarz FJ, Schnegg A: Interactions of essential metals in human physiology. Clinical, Biochemical, and Nutritional Aspects of Trace Elements. Alan R. Liss, Inc., N.Y., 1982.
43. Pollack S, George JN, Reba RC, Kaufman RM, Crosby WH: The absorption of nonfer-rous metals in iron deficiency. /. Clin. Invest., 44, 1965.
44. Aust SD, White BC: Iron chelation prevents tissue injury following ischemia. Advances in Free Radical Biology and Medicine Vol. I. Pryor, W.A., Ed. Pergamin Press, N.Y., 1985.
45. Williams ML, Shott RJ, O'Neal PL, Oski FA: Role of dietary iron and fat on vitamin E deficiency anemia of infancy. N.E.J.M., 292, 1975.
46. Whitney EN, Catolde CB: Understanding Normal and Clinical Nutrition. West, Pub., Co., N.Y., 1983.
47. Fairbanks VF, Fahey JL, Buetler E: Clinical Disorders of Iron Metabolism 2nd Ed. Gru-nard Stratton, Pub., N.Y., 1971.
48. Ibid.
49. Watts DL: Water and health. The Newsletter. T.E.I., 1, 1986. Savannah, Ga.
50. Saunders SJ, Banks S, Airth E: Iron absorption in pancreatic disease. Lancet, ii, 1962.
51. Watts DL: Nutritional relationships - Copper. 1988, (Unpub.)
52. Ibid.
53. Dexter DT, Wells FR, Agid Y, Lees AJ, Jenner P, Marsden CD: Increased nigral iron content in postmortem parkinsoniam brain. Lancet ii, 1987.
54. Dexter D, Carter C, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD: Lipid peroxidation as cause of Nigral cell death in Parkinsons Disease. Lancet II, 1986.
116